Innovative production process – prolonged clinical effect!
About


Phillex™
new generation of intradermal fillers based on stabilized hyaluronic acid of non-animal origin.
Phillex™
due to innovative method of production it was possible to obtain spherical molecules of hyaluronic acid with higher-order and dense grid .
Phillex™
first medical product with spatial ordering of molecules of hyaluronic acid (4D-matrix technology). Due to production technology of spherical molecules, PHILLEX not just removes skin defects (3D-matrix mechanism) but also allows to resist compression load (effect of "spring", 4D-matrix), which, in turn, results in greater facial tissues lifting.
Phillex™
showed its effectiveness and safety, proved by a series of comparative clinical studies conducted with Restylane (Qmed, Sweden).
Specific features: 3. Comparative diagram of pain during injection
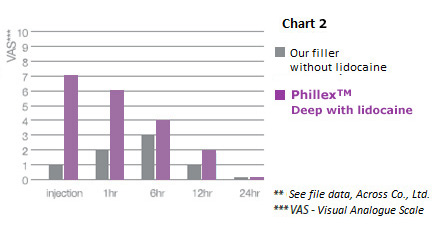
Phillex™ –contains 0.3% of lidocaine, which relieves pain suffered by the patients during or after injection; laser needle sharpening allows to minimize pain during initial injection.
Safety and efficiency
Clinical studies results, which have proven their safety and effectiveness
Clinical studies of the medical product Phillex ™ have proven their effectiveness and safety for nasolabial folds correction.
Phillex ™ does not come short to the reference medical product Restylane® (Qmed, Sweden).
| Comparative table of GAIS** results, obtained by the researcher on the 8th, 16th, and 24th week of FAS* Fig.1 | Comparative table of GAIS** results, obtained by the research subjects on the 8th, 16th, and 24th week of FAS* Fig.2 | ||||||
| GAIS** | GAIS** | ||||||
| Period of time | Number of patients | Tested product (PhillexTM Deep) | Control item (Restylane® Deep) | Period of time | Number of patients | Tested product (PhillexTM Deep) | Control item (Restylane® Deep) |
| n | Mean±SD | Mean±SD | n | Mean±SD | Mean±SD | ||
| 8th week | 66 | 2.12±0.48 | 2.08±0.51 | 8th week | 66 | 2.15±0.50 | 2.08±0.51 |
| 16th week | 66 | 2.06±0.49 | 2.02±0.51 | 16th week | 66 | 2.06±0.49 | 2.02±0.51 |
| 24th week | 66 | 1.65±0.71 | 1.64±0.72 | 24th week | 66 | 1.65±0.71 | 1.64±0.72 |
 | |||||||
Participants of the study
66 participants scored 3 or 4 points by GAIS Scale . Target: eliminate nasolabial folds.
Methodology
Arbitrary sampling; diversified blind study. Control of efficiency and safety of comparing with Restylane® (Q Med, Sweden).
Conclusions
Clinical studies have confirmed that (Deep) is safe and efficient medical product for nasolabial folds correction and does not come short to the reference medical product Restylane®.
1 Results of clinical studies made by Pronexx Co.,Ltd.
* FAS* = a set of data for a full analysis; ** GAIS ** Global Aesthetic Improvement Scale

Product range

 |  |  |  |
 |  |  | |
| Composition | Per 24 mg/ml, 0.3% lidocaine | Per 24 mg/ml, 0.3% lidocaine | Per 24 mg/ml, 0.3% lidocaine |
| Areas of injection | Wrinkles: “crow’s feet”, perioral and periorbital wrinkles, lip contour, neck wrinkles, nasolacrimal groove | Deep naso-labial folds, marionette lines, volume and contour of lips | Deep folds and wrinkles: malar region, chin, cheeks, nasal correction |
| Injection depth | Epidermis, upper dermis | Middle/deep layer of derma | Deep layer of derma/hypoderm |
| Syringe volume | 1.1 ml | 1.1 ml | 1.1 ml |
| Needle size | 30G/27G | 27G | 25G/27G |
| Storage | – | 2-25˚C | – |
| Duration of procedures | – | 12-18 months | – |
| BDDE | – | not found | – |
| Shelf life | – | 24 months | – |
Results of clinical studies proving the safety and efficiency
Results of clinical studies made by Pronexx Co.,Ltd.